Biologically Active Units
EXOSOME STANDARDIZATION AND CHARACTERIZATION

difficulties encountered in standardizing and characterizing exosomes
Despite the growing enthusiasm for employing exosomes in research and therapeutic contexts, a significant obstacle facing the field is the standardization and characterization of exosome products. In response to this challenge, exosome trailblazer Innovare Biologics® has introduced an innovative and pertinent approach to quantifying and characterizing exosomes, known as Biologically Active Units (BAU).
The aim of the Biologically Active Unit (BAU) is to standardize the assessment of nanovesicle technology, thereby facilitating its extensive adoption.
REASONING FOR INTRODUCING BIOLOGICALLY ACTIVE UNITS
The establishment of the Biologically Active Unit aimed to rectify the erroneous notion in the tissue industry that links particle count with the potency of exosome products. While this presentation employs XoGlo products as an illustrative example to elucidate the concept of BAU, this formula is applicable to any other exosome product.
The BAU accounts for the intricacies of exosomes; beyond merely the exosome concentration and volume per vial, it also incorporates the RNA concentration, a factor strongly linked to the biological activity of exosomes.
The purpose of the BAU is to establish a more effective and significant method for assessing the POTENCY of any exosome product. This stands as one of the quality attributes scrutinized for any medication, alongside Purity, Safety, Identity, and Strength.
Biologically Active Unit Formula (BAu)

- CONCENTRATION OF RNA
- EXOSOMAL CONCENTRATION
- OVERALL PROTEIN CONCENTRATION
- VOLUME OF PRODUCT PER VIAL
- (BAU) FACTOR
- Concentration of RNA
Methodology
The extraction of RNA from the exosomes is performed using a commercially available kit tailored specifically for this task.
Assessment of Purity
The RNA extraction quality is assessed through gel electrophoresis and spectrophotometric analysis, utilizing the absorbance ratio of 260 nm to 280 nm.
Quantification
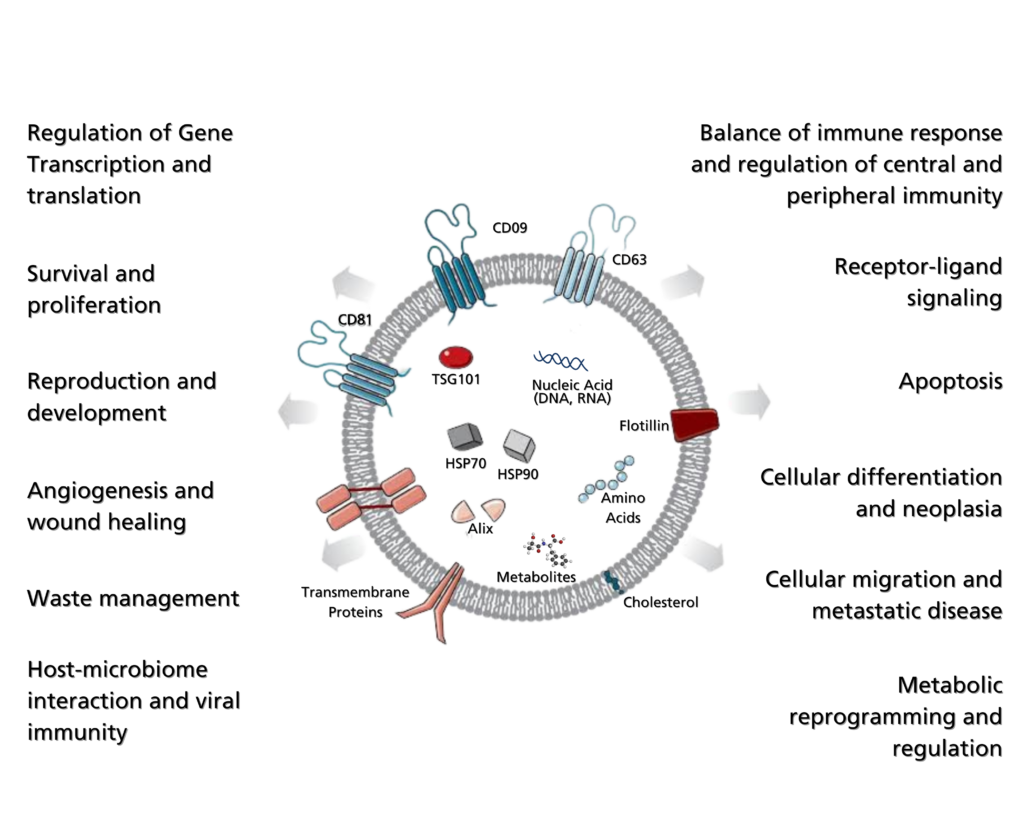
To determine concentration, a specialized RNA quantification kit is employed. This kit includes RNA standards of known concentrations to establish a standard linear regression. The fluorescence signal of the sample is compared against the standard curve to ascertain the RNA concentration.
Exosomal RNA quantity is assessed using a comprehensive RNA quantitation kit that exhibits high specificity for RNA over dsDNA. The kit incorporates RNA calibration standards with known concentrations ranging linearly from 5 to 1000 ng. Optimized for fluorescent microplate readers, the kit measures the fluorescence signal and concentration of RNA standards, plotting them on a graph to create the standard curve. This curve serves as a reference to determine the RNA concentration of unknown samples by interpolating their fluorescence signals into the linear regression. As the fluorescent signal is directly proportional to the RNA concentration, higher values correspond to higher concentrations.
- Exosomal Concentration
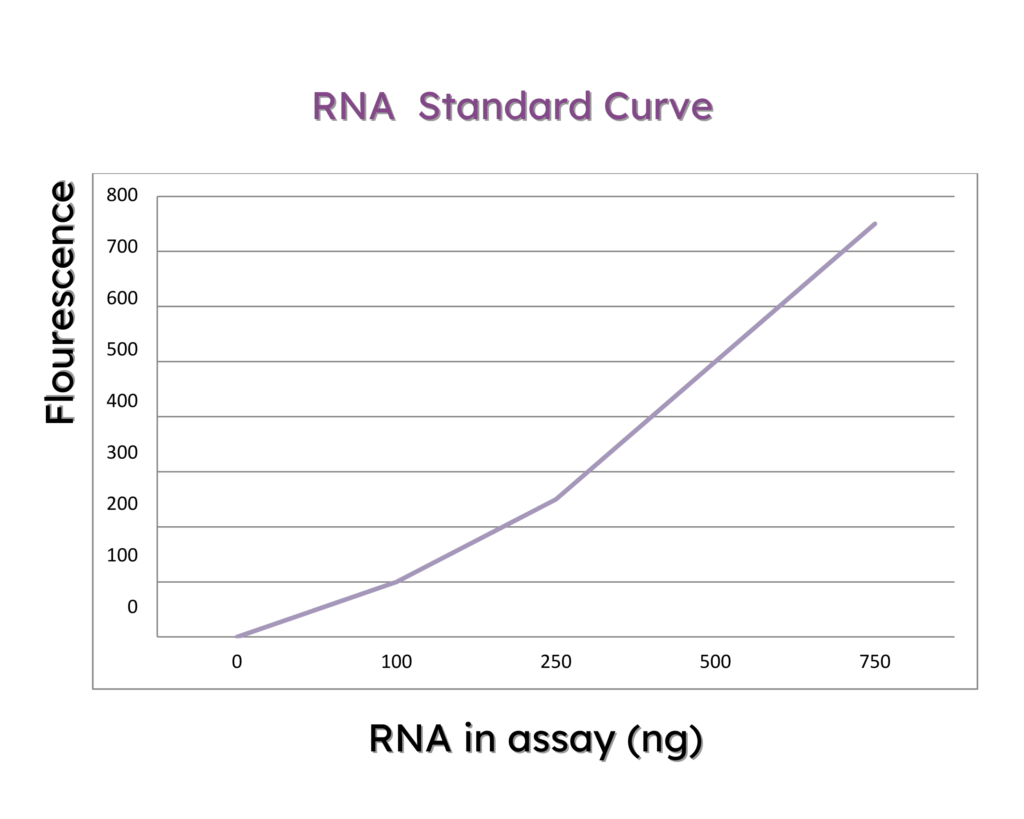
Exosome concentration is assessed via Size Exclusion High-Performance Liquid Chromatography (SEC-HPLC) utilizing Agilent 1260 equipment. In this technique, molecules are sorted based on their size or molecular weight.
Smaller molecules can penetrate the column’s pores, resulting in slower migration compared to larger molecules. Consequently, larger molecules exhibit shorter retention times (RT) in the column.
Relative Retention Time
Exosome peaks are identified by comparing their retention time to a calibration standard of known molecular weight (e.g., BSA) and concentration. This is calculated by dividing the retention time of the exosome peak by the retention time of the BSA calibration standard, which is an integral part of the system suitability.
Molecular Weight
The molecular weight of exosome peaks is determined by interpolating the retention time against a standard curve of molecular weights. This standard comprises a commercially available mixture of proteins with known molecular weights.
Concentration
Exosome concentration is calculated by interpolating the peak area of the exosome peak against a linear regression of the system suitability standard curve.
- Overall Protein Concentration
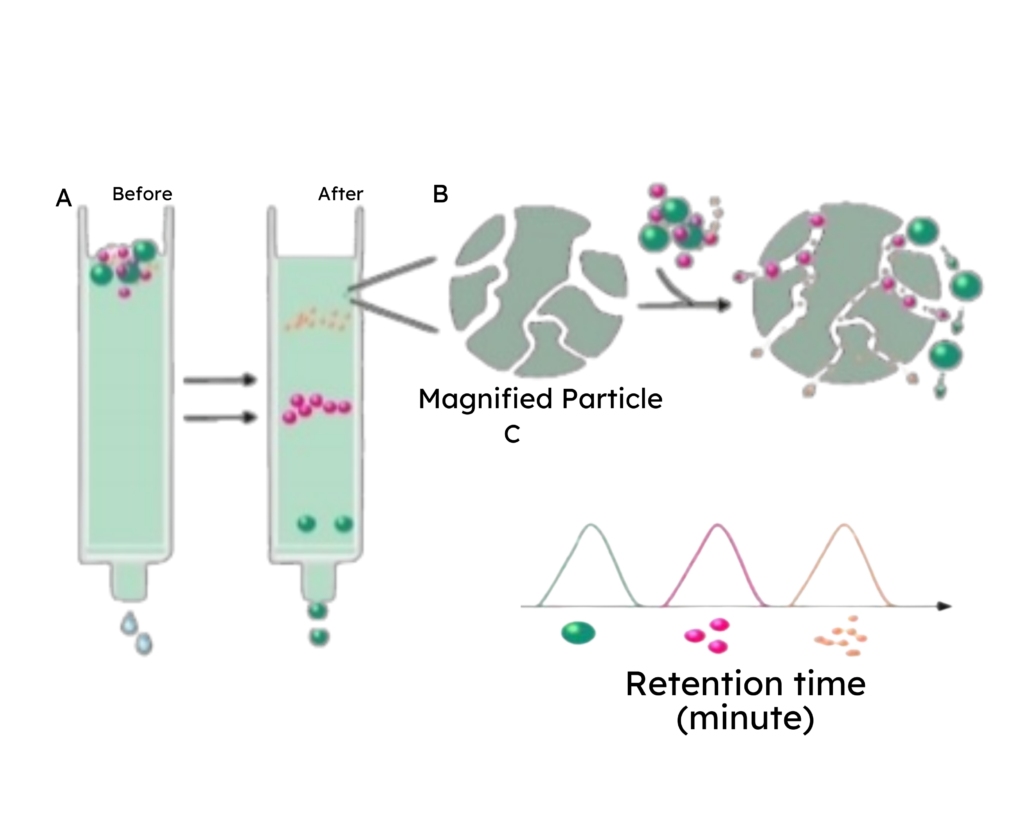
The product’s concentration is determined using spectrophotometry by measuring absorbance at 280 nm with the ID5 microplate reader. This measurement is validated through SEC-HPLC and BCA analysis.
Total protein concentration includes both exosome concentration and any additional proteins present in the product. Discrepancies in results between these quantification methods suggest the presence of components other than proteins and exosomes in the product.
Protein assays utilize standards to quantify other proteins by comparing an unknown protein quantity to known amounts of the protein standard. The absorbance and concentration of the protein standard are measured and plotted on a graph to generate a standard curve. This curve is then utilized to determine the concentration of the unknown protein.
- Volume of Product Per Vial
The vial’s volume is also factored into the equation to accommodate the various configurations of Innovare Biologics Standard and Premium offered to our clients.

- (BAu) Factor
The (BAU) factor is a fixed value established to convert experimental results to the nominal values defined at Innovare Biologics®. It serves to determine the product’s strength and will replace the values in particles as outlined below.
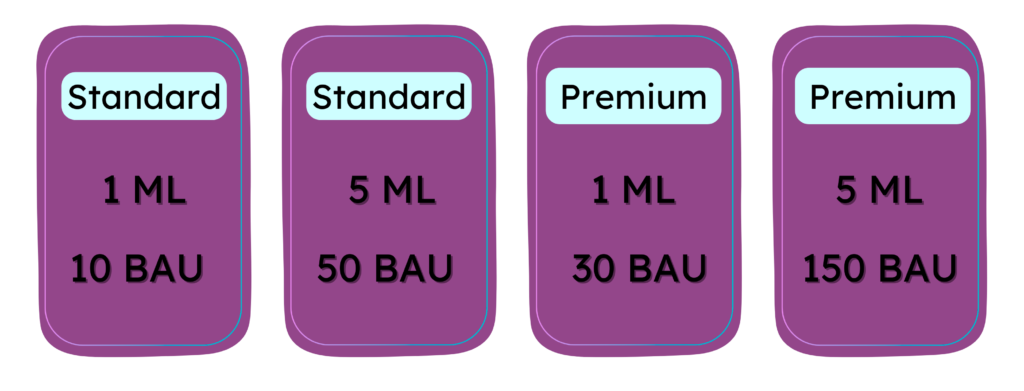
Compare the Numbers
BAU Results for Exosomes Products in the Market
